Dear Reader,
Regarding the organic peroxides at the Arkema site in Texas, I took the time and looked up the safety datasheet (SDS) of one of their products. I choose an organic peroxide product (Luperox 331M80) which a 80 % solution of a peroxide which is suitable for plastics processing.
Now after the tales of explosion in the popular media I checked the SDS for any signs of explosive behaviour, I did not see any warnings about detonation of the 1,1-di-(tert-butylperoxy) cyclohexane which is the peroxide in this product.
I also note that the product is a very long way away from being an oxygen balanced explosive. If we consider the chemistry of a detonation. It is normally assumed that an explosive such as TNT is converted to a mass of atoms, these atoms then combine to form a series of products.
The formula of the C14H28O4 if we decompose this into atoms then we would have a great deal of carbon and hydrogen which requires an oxidant to burn. If we apply the Kistiakowsky–Wilson rules then we would expect 4CO + 14H2 + 10C as the products. I suspect that the reaction would form a lot of soot and maybe some hydrocarbons. To me this does not look like a compound which has much promise as an explosive.
The formula weight of the compound is 260.374 grams per mole, I have worked out the oxygen balance of it to be -234 %, this is very far away from the values for typical explosives such as ammonium nitrate (+20 %) TNT (-74 %), nitroglycerine (+3 %) and TATB (-56 %). I suspect that such an extreme lack of oxygen together with a lack of nitrogen would lower the heat of explosion to the point at which detonation would be impossible to at least very difficult.
What the substance could do would be to generate heat in an exothermic reaction and thus burst open a container. If we consider a substance which can decompose in a reaction which has an activation energy EA. Then the rate of reaction will be given by the equation (Arrhenius).
k = A exp (- EA/RT)
Thus as A, R and EA stay the same, when T changes the rate constant will change.
If we take data from Sigma-Aldrich for the rate of decompstion of di-tert-butyl peroxide and we calculate that EA is 141124 joules per mole. While A is 5.623 x 1013 s-1.
If we consider the energy release when one mole of di-tert-butyl peroxide decomposes to 2,5-dimethyl-2,5-hexanediol then by using the difference in the heats of combustion we can work out the heat (enthalpy) of the reaction. I have worked out that the energy release when one mole of the peroxide decomposes will be 30080 joules.
If the heat capacity of both compounds is assumed to be 310 J mol-1K-1 we can work out what will happen if a infinite drum of di-tert-butyl peroxide is left at 60 oC. The drum would sit nicely doing very little for days on end, and then suddenly it will bite back in a rather nasty way. The temperature will suddenly spike. What happens is that the self heating in the drum will suddenly make it heat up to the point at which it will boil violently.
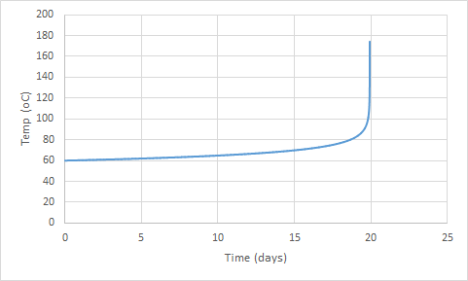
What I did was to compute the rate of the reaction at 60 degrees C, from this I worked out the heat generated by the mixture. I also worked out the amount of the peroxide which had been consumed. I assumed that the reaction rate would be constant for 30 seconds. I worked out the temperature and the peroxide concentration at the end of each 30 second time period and then used these values to start another calculation. After doing this many times I had the data for the graph.
If doubt if I have got the exactly right time between leaving the drum and the fiery exotherm, an error in the activation energy or another parameter will change the answer. What will be needed would be for someone to go and do DSC on the peroxide. compound. It is also important to note that sometimes an impurity can increase the rate of the decomposition. For example amines can increase the rate at which some peroxides decompose.
In a DSC machine a sample is heated in a small furnace in which the amount of heat energy required to increase the temperature of sample. The sample is compared with a blank (empty sample holder). In this way it is possible with a very small sample to determine if self heating can occur.
Filed under: explosives, organic peroxides, peroxides, Uncategorized | Leave a comment »


