Recently a student asked me about how to deal with the problem of making molecular orbitials in a polyatomic molecule. For example we can consider carbonyl sulfide (S=C=O). This has a sulfur, a carbon and an oxygen.
The first step of the problem is to count up the number of valence electrons which the atoms have. For it is best to have a periodic table to hand.
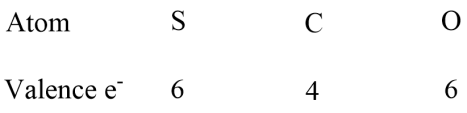
We should now make a Lewis strucutre for the molecule, we need to decide what the central atom will be in the molecule. I am sure that C=S=O will be a far higher energy than O=C=S and anyway if we look up the strucutre of carbonyl sulfide we will find that the carbon is at the centre of the molecule. We can make a first attempt at the Lewis strucutre where we have only sigma bonds and lone pairs.
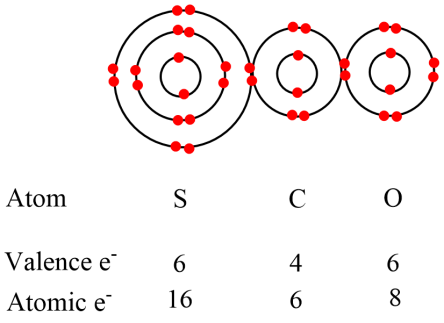
This is wrong as it has twenty (20) electrons in the outer valence shells of the atoms. We only have 16 electrons in the valence shells of the atoms. So we need to make a new attempt. In the new attempt we will have fewer electrons. I am showing the inner shells which are part of the inner noble gas cores. But for speed and ease you should leave them out.
In the new strucutre I assume that we will have a higher bond order between the carbon and the outer two atoms in the molecule.
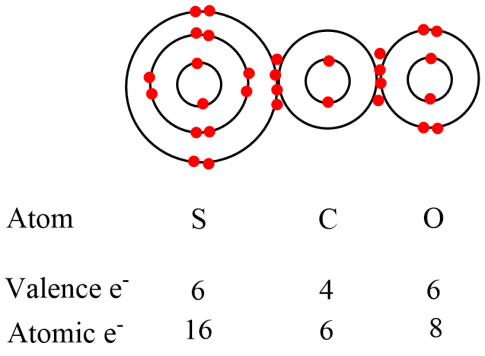
This is now right, each atom has eight electrons in the outer valence shell, the total number of valence electrons in our molecule now add up to 16 which is the sum of the number of valence electrons in the molecule.
Many years ago when I was an undergraduate people like Henry Rzepa and Derek Woollins taught me to always consider the sigma bonding first. The sigma bonds have a higher bond dissociation energy than the pi bonds. Also for the VSEPR we only consider lone pairs and sigma bonds. We should now consider the arrangement of bonds around the carbon.
We have a sum of two sigma bonds and lone pairs on the carbon. To maximize the angle between the two groups on a sphere we need a sulfur carbon oxygen angle of 180 degrees (pi radians). This will dictate that the moelcule is linear.
When we make hybrid atomic orbitials from the original atomic orbitials there is the rule that if we start with x orbitals we must end up with x orbitials. We need to have the atomic orbitials on the carbon needed to make two sigma bonds, we also know that we need to have two pi bonds to the carbon.
We have three choices with the hybird orbitial formation.
s + p + p + p makes 4 x sp3 orbitals
s + p + p makes 3 x sp2 orbitials with one p orbitial left over
s + p makes 2 x sp orbitials with two p orbitials left over.
Lets go through the options
The first one we can rule out as it would require us to have two lone pairs on the carbon and we need more electrons than are possible for the neutral molecule. We would have to have a molecule with a charge of -4. This is not reasonable. Lets leave it alone now.
The second one we need to have a lone pair on the carbon. This will force us in the VSEPR step to have a angular shape to our molecule. As real life evidence indicates that the carbonyl sulfide molecule is linear we can discount this idea. There are triatomics which are angular such as sulfur dioxide and the nitrogen dioxide radical but O=C=S is linear.
So we are left with the last option that the O=C=S molecule is linear. So at least we can decide that the central atom is sp hybridized.
The shapes of the orbitials
There are multiple levels, my coworker Christian Ekberg has the saying “There are many levels in hell”. There is no divinity or theology department at Chalmers and right now I do not have easy access to a theology prof so we will not discuss the nature of hell. But what CHE means is that there are multiple levels of understanding.
In MO theory we can go very deep but there is a level which I think is suitable for first year classes such as KTK112 at Chalmers. In this article I will restrict myself to the basic understanding. If you are a genius of MO theory and want to discuss with me the deeper part of MO theory then please feel free to contact me privately, there is a possibility that we might be able to have a shared joyous experience but I wish to bring peace and clarity to the troubled minds of first year students.
Now we need to consider the shapes of the orbitials. We can agree that there are pi systems which are involved with the carbon and also sigma bonds. We should consider the sigma bonds first.
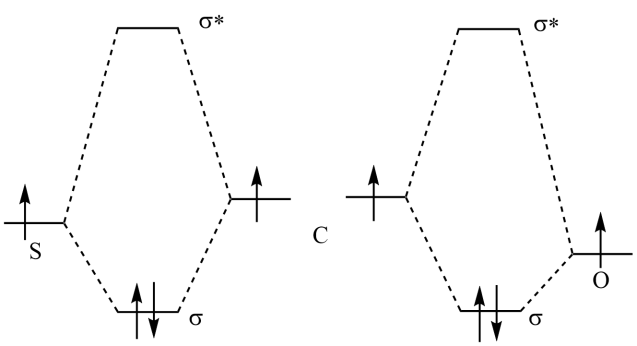
Now lets take a step back and consider a more simple example, lets consider hydrogen cyanide for a moment. We have in hydrogen cyanide sigma bonds which exist between the hydrogen and the carbon along with a bond which exists between the carbon and the nitrogen. We can make up our mind that the carbon and the nitrogen are both sp hybridized. There are two ways of looking at the problem, we have the KTK112 view of the situation as having a pair of sp orbitials on the carbon which are pointing in opposite directions and they form both bonding and antibonding orbitials with the two atoms at the ends of the molecule. Here is a MO diagram for the sigma bonds and the lone pair in HCN.
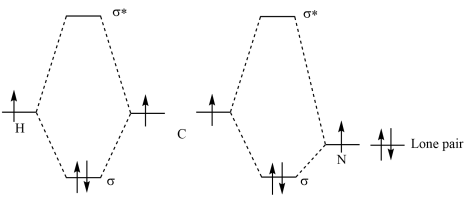
Now we go back to the O=C=S example. We can make a MO diagram for the sigma bonding only. It will look like this. We should consider the molecule as having two independent sets of sigma orbitials for the two different bonds. Here is the MO diagram for the sigma orbitials only.
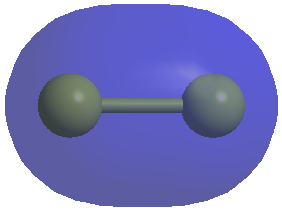
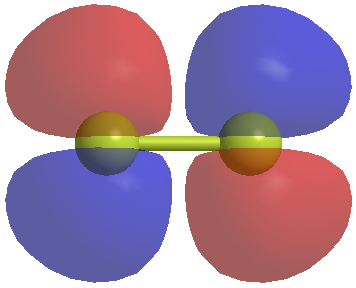
Now we have the shapes of the molecular orbitials. If we want to understand what the shapes of the sigma orbitials look like at the KTK112 level our best chance is to look at the orbitials calculated for F2. The sigma bonding orbitial between two atoms of the same electronegativity will look like this.
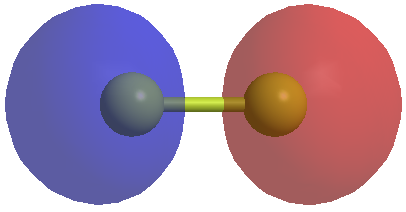
The sigma star (sigma antibonding) orbitial will look like this
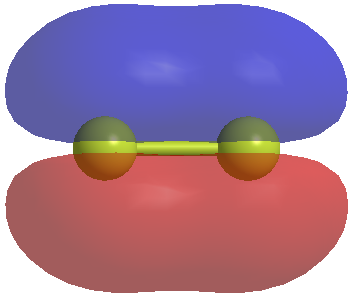
Now we should consider the pi bonding in our molecule. For the O=C=S molecule we will have pi bonds. Our central carbon will be sp hybridized so it will have two p orbitials which are normally at 90 degrees to each other. If we concentrate on the bonding between two atoms then we will have a pi system which looks like this. Firstly here is the pi bonding orbitial between two atoms.
And now is the antibonding pi orbitial associated with the two atoms.
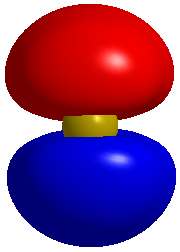
For the KTK112 level of understanding I would tell you to consider O=C=S as a molecule which has two pi systems, these will be at 90 degrees to each other. If we were to look at the molecule along the axis of the bonds then if we could see the pi clouds then we would see this for one of the pi clouds.
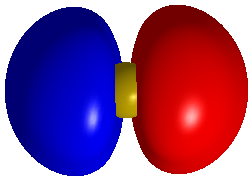
While for the other we would see this
I will write more later on the more advanced view of the bonding in the molecule.
Filed under: Uncategorized |



Go on, Have your say !