Dear Reader,
A lady from the USA who collects glass has been in communication with me regarding the uranium glass candlesticks, she has a pair of very similar candlesticks to my uranium glass one. It is possible that they all came from the same maker.
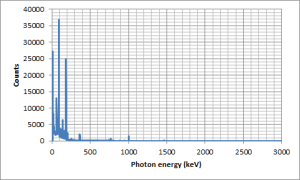
I did collect a gamma spectrum from my candlestick, as the activity level in a candlestick is low it took many hours to get a good spectrum. Here is the full range gamma spectrum from 10 keV photon energy to about 3 MeV photon energy. This covers almost all of the gamma spectrum. OK I can think of gamma events which are outside this range like the n + N-14 reaction to form C-14 which emits photons at about 10 MeV but such a high energy photon is very rare.
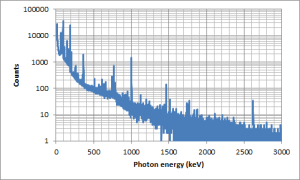
You should be able to see that almost all of the peaks are at low energies (below 500 keV), I have stretched the y axis by using a log scale so you can see some more of the minor peaks.
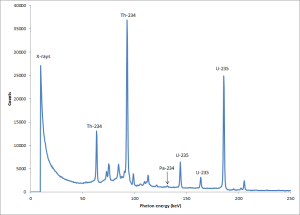
Now I have replotted the graph to show the lower energy end only, it should be clear that a series of peaks are present due to the gamma rays from the candlestick. Also on the left hand side is a big peak due to X-ray formation. What happens is that high speed electrons (both beta particles and photoelectrons) fly around inside the shielding and form X-rays by hitting the copper inner shielding on the lead castle in which the detector is placed. The copper inner shield reduces the fraction of photoelectrons which are ejected from the lead surfaces that are able to strike materials close to the detector crystal.
Also any X-rays formed in the surface layer of the lead nearest the radiation detector and the object being measured will tend to be stopped by the copper metal. By experience is that 1 mm of copper sheet is able to stop almost all copper K x-rays. And I imagine that it will stop many moderate energy photons like the moly K x-rays which are used for single crystal crystallography work and for mammograms. In the ideal world we would also have an aluminium layer inside the copper shield to further reduce the back ground at low energies due to secondary X-rays, and then a layer of a plastic like PMMA to finish the job. This is an example of graded Z shielding.
Even if the shielding is designed in such a way to prevent X-rays reaching the detector, it is possible to create X-rays inside the sample. The ideal sample for gamma counting would be a very small sample with a very high specific activity (radioactivity per mass or unit volume). If this was wrapped in plastic then any beta particles which come from the sample would lose their energy gently in the plastic without making horrible X-rays. But in our case the volume of the sample is large and the glass contains plenty of elements such as silicon which can form bremsstrahlung as the electrons pass close the atomic nuclei.
But we will not let a little bremsstrahlung spoil our fun. Here is the lower end of the gamma spectrum.
What we need to do next is to work out the relative efficiency for the detector for the different gamma lines, what we need to do is to measure the size of the peaks which we can identify and then use the fact that the yield of the different gamma lines are known. This will allow us to reconstruct the relative efficiency for the detector. In case anyone is thinking that we could do it with more easy by using a mixed photon energy radioactive source such as a radium-226 source. There is a problem, the odd shape of the sample makes it have a sum of many different counting geometries and a lot of self adsorption for the lower energy gamma photons.
I could make it more simple by crushing or melting my candle stick to make it into a more simple shape, but that would spoil my candle stick so I am not going to do that.
Filed under: Uncategorized |



Go on, Have your say !