Dear Reader,
I have been asked to explain the dire event which occurred this week in Beirut, what happened was that thousands of tons of ammonium nitrate stored in a dockside warehouse exploded after a fire broke out.
My view of the event is that we are facing the problem of the DDT (deflagration detonation transition).
This is an important thing to understand in energetic materials, it is possible for a single substance or composition to react in both a deflagration and a detonation.
In a deflagration a reaction front moves through a mass of material by means of heating, as one part of the mass reacts it heats the next part of the mass. This propagates the reaction in a much slower way than a true detonation which is propagated by a supersonic shockwave which passes through the mass.
It is important to also note that for many explosives if they are burning under pressure that the higher the pressure the faster, they react. It is important to keep in mind that if a container contains a mass of an explosive then if the explosive is ignited with heat then the pressure caused by the gases generated by the reaction as they exit through a small hole will generate pressure. This increase in pressure can make the reaction rate at the surface of the explosive increase.
It is possible to reach a tipping point at which rate of reaction becomes so fast and the pressure so large that the remaining bulk of the explosive starts to be initiated by a shock wave which outpaces the thermal propagation of the reaction. At this point the reaction has undergone the DDT.
The far greater rate of gas production and heat release will now make the physical effects of the reaction far more devastating.
I am aware of a firearms accident which destroyed an elephant gun, I never saw the gun and I do not know the person who had it. It might be an old wives tale but the chemistry / physics of it is plausible. What happened was there was a person who was hunting dangerous large animals with some scary monster high powered rifle. What happened was it was a bolt action rifle which had a magazine.
With some bolt action designs, which includes some WW I rifles, it is possible to pull back the bolt and then add more cartridges to the magazine while the magazine is inserted into the rifle. The story was that the hunter kept on shooting and then topping up by adding more from the top.
What happened was that each time the gun was fired the almighty recoil slammed the bullet a little further back into the brass case than it should have been.
Eventually the misguided hunter went and fired the cartridge which was at the bottom of the magazine.
The firing pin falls on the primer which then ignites the propellant, the propellant in a shotgun shell, rifle cartridge, pistol cartridge or even a giant artillery gun is supposed to deflagrate giving the projectile a prolonged shove as it races faster and faster along the barrel.
But when the propellant was inside too small a volume (pushing the bullet too far back into the case lowers the free space inside the case) the rate of burning became too high. This speeded up the burn rate which then caused the pressure in the case to build up to some level which the gun was never intended to cope with.
The gun then broke apart, I have no idea what happened to the person holding it. Some firearms accidents where guns break apart in use can prove fatal. I have seen some horror photos of guns which have failed in use due to things like mistakes in ammunition production. I have seen photos of revolvers where the block of metal which holds the cartridges has split apart the photos, I have seen of rifles which have failed are scarier. My reasoning is that when firing a revolver, you have it at arm’s length away from your head and torso, with some luck the rain of metal fragments will fly sideways away from you.
With a rifle you are likely to have the breach close to your chin thus putting your tender bits rather closer to the event.
But back to the ammonium nitrate explosion, we have considered how a defective (or abused) cartridge can blow up a gun. There is another way in which a normally placid substance can change from a friendly pussycat into a raging tiger with a dire attitude problem.
If I was to place a small amount of an energetic material on a smooth concrete floor and then ignite the pile, then it will burn smoothly in open air. There will be lots of sparks, smoke and maybe a flash.
But if we make the pile bigger and bigger then the outside of the pile will burn creating gas pressure which compresses the core of the heap. If we were to make the heap sufficiently large, then we could have a DDT which would then result in a gentle fire suddenly transforming into a mighty explosion.
I think that during the early stages of the event in Beirut that a fire started in the warehouse, I have seen reports that welding was being done on the building during repairs. This is one of the big errors which I see in this case.
I hold the view that hot work should as welding should not be done in a building packed with either a flammable or explosive substance. If I had been in charge of the site I would have insisted that the workers either empty the building and then do the hot work OR find a method of repairing the building which does not require hot work to be done.
For example, when making a roof it is possible to get tarred sheets which are sealed by means of heating them with a gas torch and there is also another type of sheets which do not require this heating. I would argue that the latter type is a safer type to work with near flammable or explosive materials.
I have seen reports that the welding was being done to plug a hole in the building, one option I see would be to use welding to build a new section of wall. But do the welding somewhere else on the site and then move the new section to the building and then bolt it onto the building thus fixing the hole. While it is possible to make some sparks when ferrous metal touches either stones or other ferrous metal, I think that bolting two bits of metal together is safer than using welding equipment.
A well-designed explosives / petrol / flammables store should be free of ignition sources, this includes making sure that the electrical fittings in the building are not going to ignite things. I would want to include some means of lighting inside the store which is sealed or otherwise guarded to prevent an explosion. One common trick I have seen on solvent stores is to put the light switch on the outside of the building, the idea is that if the switch is going to make sparks then it is better to put it outside the building rather than inside it.
Another step I would have wanted to take would be to limit the amount of ammonium nitrate in the building. Ammonium nitrate is classified as 1.5, this is a rather special class of explosives. The normal classes are 1.1, 1.2, 1.3 and 1.4.
| Class |
|
|
|
|
|
| 1.1 |
Mass detonation possible |
Bulk storage / transport of TNT |
| 1.2 |
Projectile hazard |
Typical hand grenades |
| 1.3 |
Fire hazard, minor blast / projectile hazard |
Firework rockets |
| 1.4 |
Minimal hazard |
A box of garden fireworks |
| 1.5 |
Mass detonation possible but very insensitive |
Ammonium nitrate |
| 1.6 |
Very insensitive, mass detonation impossible |
|
This means that while it is possible to create a detonation with ammonium nitrate it is normally very difficult to do so. The IRA used to use ammonium nitrate mixed with fuel oil (ANFO) for large bombs. To make such a bomb explode the ANFO must be subject to an almighty shock, I would rather not discuss the topic of how to build a bomb out of ANFO. Do not bother me by asking me, the only answer you will get is “No comment” or some swearing if you are very unlucky.
But once a mass of a secondary explosive such as TNT or ammonium nitrate has been provoked into detonation it is possible for the shock wave of the detonation to trigger other masses of explosives. There is a munitions ship which sank in the Thames estuary (SS Richard Montgomery) which is packed with explosives, it is feared that if one bomb inside this wreck was to detonate it could cause other bombs to detonate as well this could result in a large explosion which could cause a lot of damage.
One method of mitigating against a vast explosion is to split the explosive store into different areas, if the heaps of the ammonium nitrate were sufficiently spread out that the detonation of one could not trigger another then the worst case event would be made smaller. As blast obeys an inverse square law if the ammonium nitrate had been stored in four equal amounts then the distance at which a given effect occurs would be halved, this would greatly reduce the number of square miles over which the horrible off site consequences would be experienced.
There is the problem that if a series of warehouses in different parts of the city / country were built which each contained a quarter of the total stockpile that it would increase the security cost of keeping the material away from malefactors. It could be argued that the security cost will be proportional to the perimeter of the explosives storage area.
We also have the also the problem that if the explosive was more spread around the city that if the malefactors were to trigger detonations as an act of terror then they might stand to cause more damage. As blast effects obey an inverse square law with the distance from the explosion but the energy delivered to a surface by an explosion is proportional to the amount of explosive.
The surface area of a sphere is given by the equation
If we assume that the explosion occurs on the surface of a flat earth then if energy deposited on the surface of a hemisphere between ground level and 100 meters up into the air matters then we can consider the fraction of the blast energy which is lost into space / the air.
If we have a perfect sphere with radius r, then the circumference at latitude (q) is given by the following equation.
The surface area of the part of a sphere which is north of a given latitude is given by
The blast effect of an explosion can be predicted using the following equation where k is a constant, q is the yield and r is the distance from the explosion.
Now if we rearrange the formula we can get the distance r if we keep b and k constant and alter q.
If we assume that a blast which is at value b or higher will destroy buildings or kill people then if this value of b is set at 1, then when k = 1 then when q is 1000 then r will be 31.62 km and at the edge of the zone of destruction 0.0003162 of the energy of the explosion will be in the 0 to 100 m high range then the blast will deliver 3.16 units of energy into this important strip around the hemisphere.
If we reduce the yield (q) to 250 then r will become 15.81 km and then the explosion will deliver 0.006324 of its energy into the important strip, thus 1.58 units of energy are used to cause damage at this distance. Thus we can see how the quarter scale explosion is twice as able to use its energy to cause damage.
With much smaller explosions this effect is not very important, but at the upper end of the explosion yields it does become more important. I think that normally we need to consider this loss of energy into space normally only for nuclear bombs and large rocks which crash into the earth from space. With nuclear bombs and the rocks from space often the explosion occurs in the air (air burst) rather on the surface of the earth. For an air burst at a very high height such as starfish prime test or the test of the air genie (John in operation Plumbbob) the fraction of the energy which is lost into space is very high as the event occurs far above the earth’s surface.
With a curved earth this effect is even greater but the maths is a bit more complex. This also explains why MRV / MIRV systems have been deployed on ICBMs. I would rather not write “use” as use suggests something does something useful, I think that blowing up cities is never a “useful act”. The idea of MRV technology is to launch a single ICBM which then releases several nuclear bombs which then land at different points. The reasoning is that if the area being attacked is defended by an antiballistic missile system then the defensive system will be more likely to be overwhelmed by the number of objects approaching and also if all the warheads get through then it will cause more damage for a given total explosive yield than a single warhead would.
A solution which avoids creating the giant ANFO version of a MRV attack on the city is to put all the ANFO at a single site but in multiple piles. Each pile should be separated from the other piles by a mound of earth or some other suitable object which will prevent blast and projectiles from one pile exploding triggering another pile. In this way a compact and safer explosives store can be built.
Another issue I see on the news is that there are reports that fireworks were being stored in the same building as the ammonium nitrate. I checked the international rules on explosives, bulk ammonium nitrate is in compatibility group D while fireworks are in group G. Group D can be defined as
“Secondary detonating article containing a secondary detonating explosive substance without means of initiation and without a propelling charge”
While group G is
“Pyrotechnic substance, or article containing a pyrotechnic substance, or article”
The UN rules state that classes D and G can be stored / transported together but this is “at the discretion of the national competent authority”. I understand this as meaning “you can do it BUT you first need to argue a good case to the national explosives safety authority and get them to give you permission”.
I do not think that putting nylon bags of fireworks which customs have seized in the same building as a bulk storage of ammonium nitrate is wise. My worry is that the fireworks are easy to ignite, the fireworks could ignite and then the fire could spread to the ammonium nitrate. Also if the fireworks are in class 1.3 or worse still in 1.2 then they could hinder firefighting efforts thus making it harder to control a fire which is endangering the ammonium nitrate store.
My gut feeling is that illegal fireworks should be kept in a separate area of the site well away from the ammonium nitrate. I also hold the view that as soon as any court case which revolves around the fireworks is over then they should be disposed of. There are various options which work for the safe disposal of fireworks. While it might seem primitive burning them inside cages is a far better method of dealing with them than the dire method used by Donaldson Enterprises in Hawaii (2011 accident).
What happened in Hawaii was that fireworks were seized by the customs authority after someone attempted to import display fireworks (1.3) claiming that they were in the lower hazard class (1.4). The problem was that the fireworks could not be sold on the open market and thus they needed to be disposed of. The task was given to a company who then did the work in a very dangerous way. I think that the system of work changed fireworks which were in class 1.3 into material which was in a higher hazard class. The accident site also had various ignition sources in it which should have been absent.
Filed under: Uncategorized | 1 Comment »



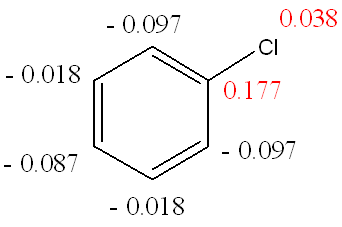 The charges on the non hydrogen atoms of chlorobenzene
The charges on the non hydrogen atoms of chlorobenzene